Services
We use state-of-the-art serialization equipment from one of the industry leaders — Antares Vision Group.
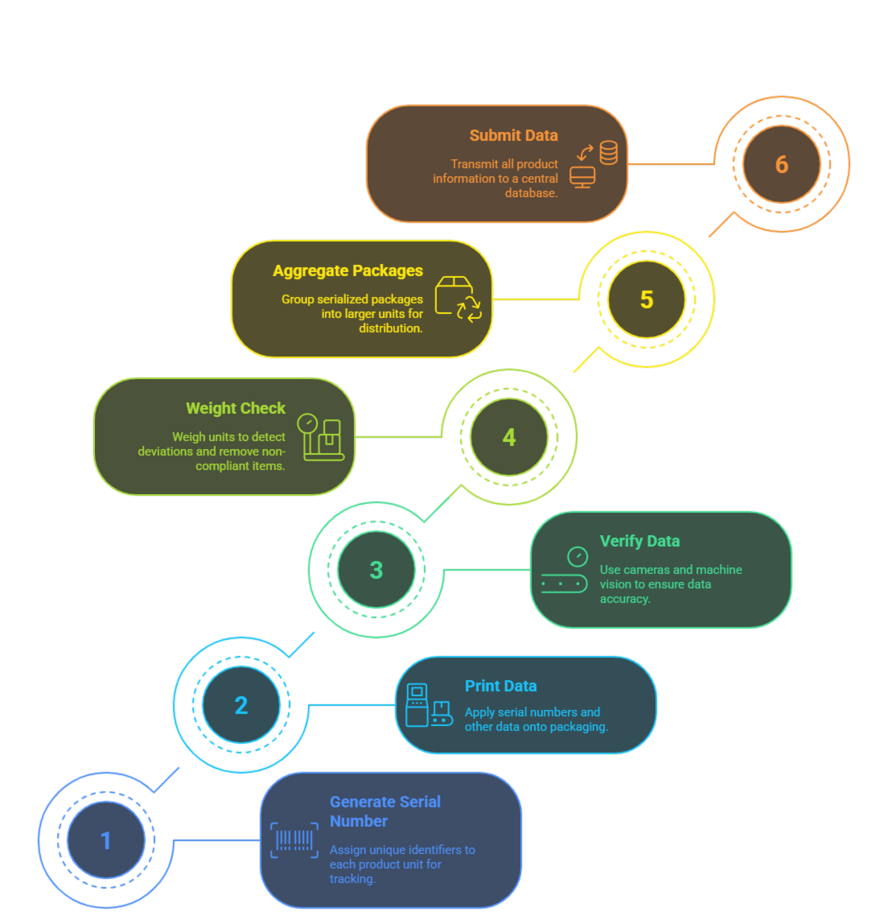
The serialization process within our Track & Trace system consists of several key stages, ensuring full traceability of products throughout the entire production and distribution chain.
Generation of a Unique Serial Number:
Each product unit is assigned a unique serial number used for identification within the system. These numbers are generated in accordance with regulatory requirements and Track & Trace standards.
Data Printing:
The serial number and other required information (such as GTIN, manufacturing date, and expiration date) are printed on the packaging using printing technologies such as:
TIJ (Thermal Inkjet Printing) — for clear and fast application of data.
Data Verification:
After printing, the information is verified using high-precision cameras and machine vision systems, which:
Recognize the serial number and other printed data.
Assess the print quality.
Detect any errors or defects.
Weight Check and Rejection
Each unit is weighed to detect any deviations.
Non-compliant units are automatically removed from the production flow.
Aggregation:
After serialization, individual packages can be grouped (e.g., into cartons or pallets). Each group is assigned a unique identifier that links it to the serialized units it contains.
Data Submission to the Track & Trace System:
All information on the manufactured products is transmitted to a central database for further monitoring and reporting to regulatory authorities.
Key Advantages of Our Serialization System:
Scalability and flexibility in packaging formats (from small boxes and bottles to larger formats).
Tool-free automated format adjustments.
Minimal downtime thanks to quick print parameter changes.
Full compliance with international Track & Trace requirements.
This system ensures reliable protection against counterfeiting, supply chain transparency, and full compliance with regulatory requirements.
The serialization process within our Track & Trace system consists of several key stages, ensuring full traceability of products throughout the entire production and distribution chain.
Generation of a Unique Serial Number:
Each product unit is assigned a unique serial number used for identification within the system. These numbers are generated in accordance with regulatory requirements and Track & Trace standards.
Data Printing:
The serial number and other required information (such as GTIN, manufacturing date, and expiration date) are printed on the packaging using printing technologies such as:
TIJ (Thermal Inkjet Printing) — for clear and fast application of data.
Data Verification:
After printing, the information is verified using high-precision cameras and machine vision systems, which:
Recognize the serial number and other printed data.
Assess the print quality.
Detect any errors or defects.
Weight Check and Rejection
Each unit is weighed to detect any deviations.
Non-compliant units are automatically removed from the production flow.
Aggregation:
After serialization, individual packages can be grouped (e.g., into cartons or pallets). Each group is assigned a unique identifier that links it to the serialized units it contains.
Data Submission to the Track & Trace System:
All information on the manufactured products is transmitted to a central database for further monitoring and reporting to regulatory authorities.
Key Advantages of Our Serialization System:
Scalability and flexibility in packaging formats (from small boxes and bottles to larger formats).
Tool-free automated format adjustments.
Minimal downtime thanks to quick print parameter changes.
Full compliance with international Track & Trace requirements.
This system ensures reliable protection against counterfeiting, supply chain transparency, and full compliance with regulatory requirements.
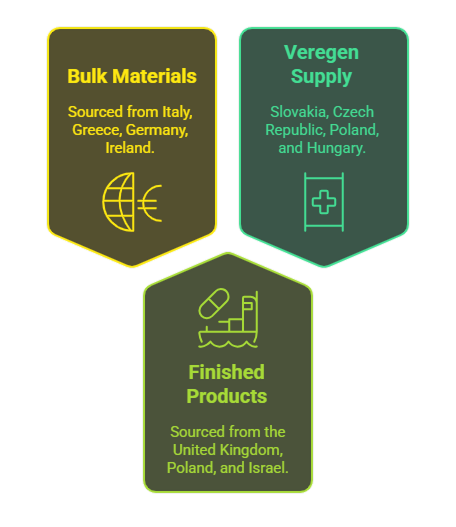
Our company sources both bulk materials and finished pharmaceutical products from various countries, including Italy, Greece, Germany, Ireland, the United Kingdom, Poland, and Israel.
We carry out serialization of the products and organize their delivery to the EAEU countries, where we have an extensive partner network and broad distribution capabilities.
In addition, we are the Marketing Authorization Holder (MAH) for the medicinal product Veregen, and we will be supplying it to Slovakia, Czech Republic, Poland, and Hungary.
In the near future, we plan to significantly expand our product range for distribution across the European market.
We also plan to carry out serialization in-house for supply to EU countries.
We carry out serialization of the products and organize their delivery to the EAEU countries, where we have an extensive partner network and broad distribution capabilities.
In addition, we are the Marketing Authorization Holder (MAH) for the medicinal product Veregen, and we will be supplying it to Slovakia, Czech Republic, Poland, and Hungary.
In the near future, we plan to significantly expand our product range for distribution across the European market.
We also plan to carry out serialization in-house for supply to EU countries.
In addition to distribution, our company provides pharmacovigilance services and regulatory support both in the European Union and the EAEU.
SWITCH-STOR LLC:
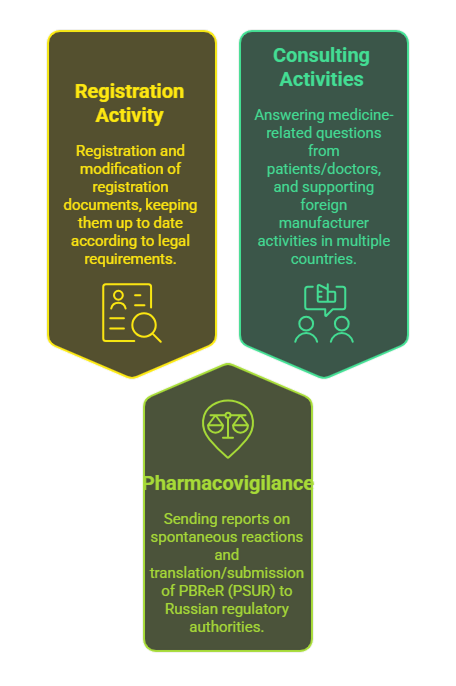
A) Registration activity:
— Registration and modification of registration documents on the territory of the Russian Federation and other countries;
— Keeping the registration documents up to date (in accordance with the applicable legal requirements).
B) Pharmacovigilance:
- Sending reports on spontaneous reactions to the regulatory authorities of the Russian Federation;
- Translation and submission of PBReR (PSUR).
C) Consulting activities:
- Answers to questions from patients and doctors related to medicines;
- Full support of the activities of a foreign manufacturer on the territory of the Russian Federation, Ukraine,the Republic of Uzbekistan, the Republic of Armenia,
the Republic of Belarus, the Republic of Kazakhstan and the Kyrgyz Republic.
SWITCH-STOR LLC:
A) Registration activity:
— Registration and modification of registration documents on the territory of the Russian Federation and other countries;
— Keeping the registration documents up to date (in accordance with the applicable legal requirements).
B) Pharmacovigilance:
- Sending reports on spontaneous reactions to the regulatory authorities of the Russian Federation;
- Translation and submission of PBReR (PSUR).
C) Consulting activities:
- Answers to questions from patients and doctors related to medicines;
- Full support of the activities of a foreign manufacturer on the territory of the Russian Federation, Ukraine,the Republic of Uzbekistan, the Republic of Armenia,
the Republic of Belarus, the Republic of Kazakhstan and the Kyrgyz Republic.
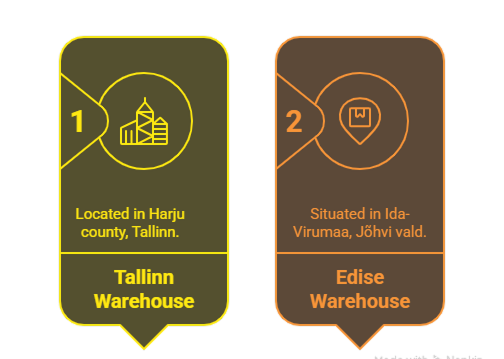
We operate two warehouses in Estonia:
One in Tallinn (Eesti, Harju maakond, Tallinn, Haabersti linnaosa, Härgmäe tn 22, 13525) and another in Edise, Ida-Virumaal (Eesti, Ida-Viru maakond, Jõhvi vald, Edise küla, Aiandi keskus 7, 41558)
One in Tallinn (Eesti, Harju maakond, Tallinn, Haabersti linnaosa, Härgmäe tn 22, 13525) and another in Edise, Ida-Virumaal (Eesti, Ida-Viru maakond, Jõhvi vald, Edise küla, Aiandi keskus 7, 41558)
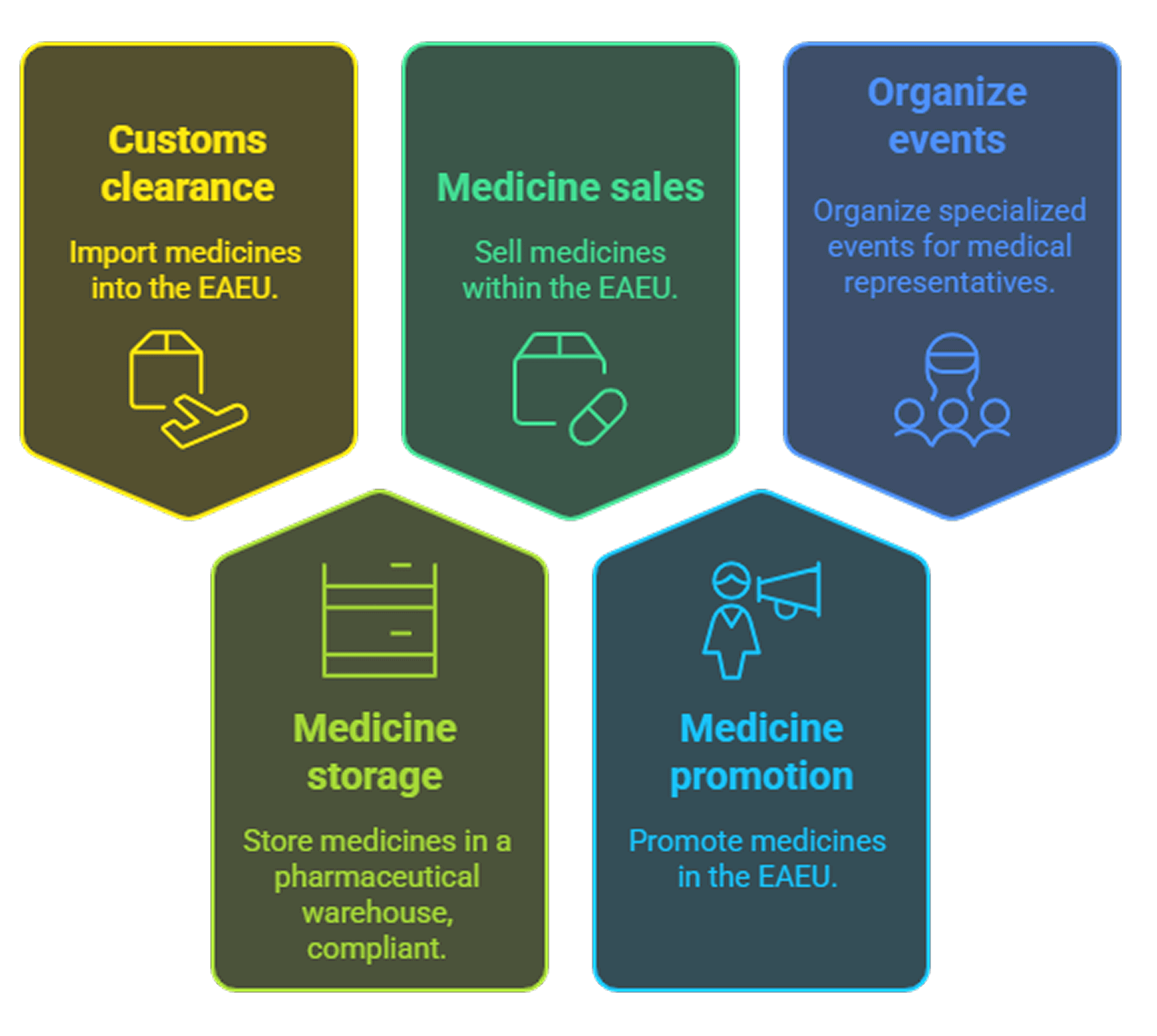
SWITCH-STOR LLC:
Implementation of customs clearance when importing medicines into the territory of the EAEU.
Storage of medicines in your own pharmaceutical warehouse in compliance with the
necessary storage conditions (the required temperature regime for medicines).
Sales of medicines on the territory of the EAEU.
Implementation of the promotion of medicines on the territory of the EAEU.
Organization of events (discussion clubs, congresses, conferences and other specialized events) for representatives of medical and pharmaceutical organizations in order to promote medicines in the pharmaceutical market of the EAEU.
Implementation of customs clearance when importing medicines into the territory of the EAEU.
Storage of medicines in your own pharmaceutical warehouse in compliance with the
necessary storage conditions (the required temperature regime for medicines).
Sales of medicines on the territory of the EAEU.
Implementation of the promotion of medicines on the territory of the EAEU.
Organization of events (discussion clubs, congresses, conferences and other specialized events) for representatives of medical and pharmaceutical organizations in order to promote medicines in the pharmaceutical market of the EAEU.


